
• Innovation Reinvented
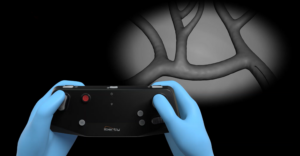
LIBERTY® Robotic System
LIBERTY is the first ever single-use endovascular surgical robotic system designed to streamline endovascular procedures.*
Endovascular Market Today
The endovascular today is a massive market with unmet needs that can be met with Robotics, however, the penetration of existing robotics is extremely low due to many barriers preventing adoption.
Over 5M+ Procedures in the US Alone¹
Only <1% of Endovascular Procedures are done Robotically²
Dedicated Room
& Staff
Large & Expensive
Capital Equipment
Time Consuming
Setup & Use

Long Learning
Curve
>75% of Interventionalists Plan for Robotics to become a part of their practice.³
In the next 3-5 years the majority of interventionalists expect to be using Robotics³, but there currently exists nothing to overcome the multiple barriers leading to the low penetration of them in the Endovascular space.
No Data Found
24% No, doesn’t expect to personally use
16% Yes, within 1 year
30% Yes, within >1-3 years
30% Yes, within 3+ years
Eliminating Barriers, Allowing Access
Our mission is to break down barriers to access through single-use robotic technology focused on the Endovascular space.

- No upfront capital expense
- Single-Use
- Aims to increase procedure efficiency
- Reduce exposure to radiation*
- Eliminate user physical strain*
*When operating seated away from radiation source
- No dedicated infrastructure required
- Can be utilized in multiple sites of service
- Designed to use off-the-shelf instruments
I believe my fellow interventional radiologists, as well as the hospital administrators, will gravitate to the LIBERTY Robotic System when it becomes commercially available because I believe it addresses many clinical and financial needs…

Vincent Vidal
Timone HospitalHaving completed over 100 robotic cases in the interventional space using larger bulky capital equipment, I was immediately drawn to the LIBERTY technology and the barriers it breaks down…

Ripal T. Gandhi
Miami Cardiac & Vascular Institute…As someone who does many interventional oncology procedures, I was impressed with how easy the system was to set up and how effortlessly I could get to my target.

Thierry Jacques De Baère
Institut Gustave Roussy Cancer CenterThe LIBERTY Robotic System intrigued me from the first time I saw it, due primarily to the future I feel robotics will play in our field.

David C. Madoff
Yale Medical Center¹1Medtech 360 Reports. ²Deduced from Public Records. ³Survey of 200 Interventionalists, data on file.
*LIBERTY is still under development and is not currently available for sale in the USA or any other jurisdiction
Our Offices
Massachusetts
Microbot Medical Inc.
288 Grove St Suite 388
Braintree, MA 02184
Phone: +1 781-875-3605
Email: info@microbotmedical.com
Israel
Microbot Medical Ltd.
6 Hayozma St.,
Yoqneam, P.O.B 242 IL 2069204
Phone: +972-4-8200-710
Fax: +972-4-8200-712
Email: info@microbotmedical.com


